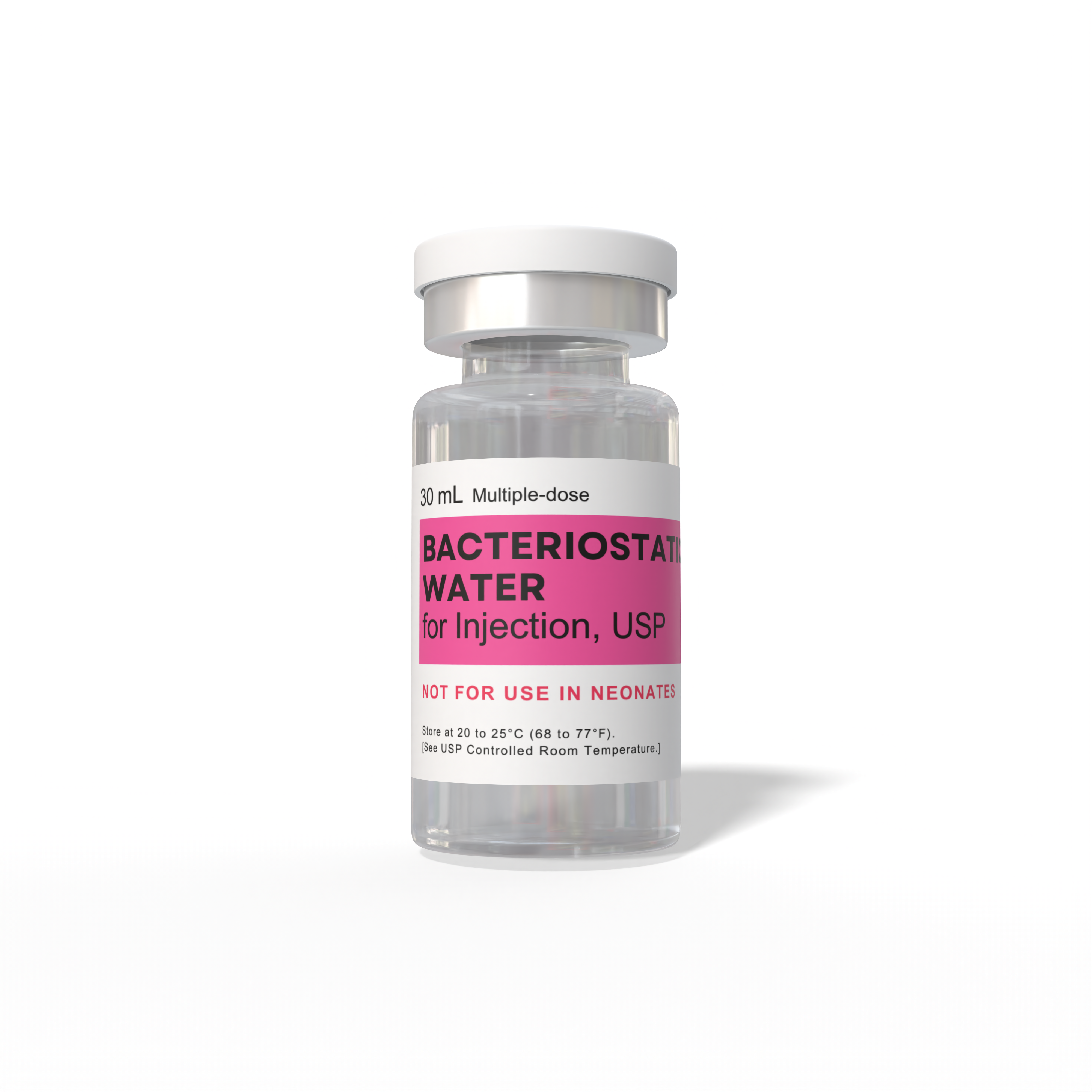
Bacteriostatic Water

Proper Handling
- Specifications: Multiple-dose Bacteriostatic Reconstitution Water for injections
- Storage: Store at 20 to 25°C (68 to 77°F) USP Controlled Room Temperature
- Expiration: After opening, it is recommended that you discard after 28 days of its initial use
Questions?
Schedule a consultation with a practitioner specialized in peptide therapy, who will determine the best plan for you.
Bacteriostatic water, USP, isn’t your average H2O; it's a sterile water solution with a small amount of 0.9%- 1.1% benzyl alcohol, acting as a bacteriostatic agent. This specialized composition makes it a go-to choice for diluting or dissolving medications for various types of injections. Its ability to halt bacterial growth ensures the solution remains sterile over multiple uses, distinguishing it from other water solutions in medical and research settings.
This product is sold for research purposes only, unless prescribed or handled by a licensed physician. This product must be stored at room temperature away from sunlight. Dispose 28 days after its initial use.
Please refer to our terms and conditions prior to purchase.
Safety Information: Keep this product out of the reach of children. This product is for use and handling only by persons with the knowledge and equipment to safely handle this material. You agree to indemnify us for any adverse effects that may arise from improper handling and/or consumption of this product.
The articles and information on products that may be found on this website are provided exclusively for the purposes of providing information and education. These items are not pharmaceuticals or medications, and the Food and Drug Administration has not given permission for the treatment or prevention of any disease, medical condition, or ailment using them.
Frequently Asked Questions
Bacteriostatic Water
-
Bacteriostatic water is a solution of sterile water and benzyl alcohol. This solution is used to create a solution of various medications for the application of that medicine through injection.
There are various uses for bacteriostatic water in medical and research applications, such as dilution, or as a solvent. All of these situations require a solvent that carries zero microbial load and acts as a preventative against microbial growth. -
Reconstituting peptides refers to the process of dissolving or rehydrating lyophilized (freeze-dried) peptides to prepare them for use.
This step is required because peptides cannot exert their biological activity in a solid state and must be reconstituted back into a liquid state.
Most peptides are also in a liquid state when they are initially synthesized and manufactured. However, liquid formulations tend to be unstable due to their physical and chemical degradation susceptibility. This is why manufacturers turn them into a solid state via various methods, such as freeze-drying.
Freeze-drying is also known as lyophilization and turns the peptide into a dry, powder form to enhance its shelf life. A lyophilized peptide powder is more stable and can be stored for longer periods without degradation. Lyophilization helps preserve a peptide’s integrity and activity, making it easier to handle, store, and transport.
-
Peptides are commonly supplied in a lyophilized (freeze-dried) powder form. Lyophilized peptides commonly resemble a small white “puck” that may have a fluffy or more granular appearance.
Before lyophilized peptides can be utilized, they must be reconstituted for bioavailability; they have to be dissolved in a liquid solution.
For instructions, schedule a consult to speak with a specialist. They will assist you with proper handling, storage, and any questions you may have.
-
Bacteriostatic water does go bad.
It is recommended that after you open, or use a vial of bacteriostatic water, that you dispose of it after 28 days. This is because the effectiveness of benzyl alcohol as a preservative loses its potency with repeat use and exposure.
This can cause bacteria to thrive inside the vial. Since many bacterial infections are largely invisible to the naked eye, it’s simply best practice to throw it away 28 days after its initial use.If left unused and unopened, bacteriostatic water can be stored long-term.
-
Bacteriostatic Water is indicated only for diluting or dissolving drugs for intramuscular or subcutaneous injection, according to instructions of a healthcare provider or manufacturer.
Follow the recommended dosage and administration instructions provided by the provider. Avoid self-dosing or altering dosages without professional guidance.
-
Storing Bacteriostatic Water is simple, because it does not require refrigeration.
- Find a relatively cool location, between 60 - 80 degrees Fahrenheit (room temperature).
- Make sure this location is kept out of sunlight, preferably a cabinet or a drawer.
This can increase the shelf life of the vials for months and potentially years.
-
A common misconception is equating bacteriostatic water to normal saline.
- Bacteriostatic water is excellent for dissolving or diluting medications
- Saline is a champ for rehydration and electrolyte balance restoration, with its salt concentration mirroring that of bodily fluids.
- Bacteriostatic water boasts 0.9% benzyl alcohol, while normal saline contains 0.9% sodium chloride.
- Bacteriostatic water is for diluting or dissolving medications, whereas normal saline is your go-to for rehydration and electrolyte balance.
The main difference between sterile water and bacteriostatic water is due to the absence of a bacteriostatic agent. This absence earmarks sterile water for one-time use, unlike bacteriostatic water, which remains usable over a duration. The lack of a bacterial growth inhibitor in sterile water mandates its disposal post a single use to avert microbial contamination.
-
We're happy to help answer questions you may have, or refer you to a licensed healthcare professional for medical advice. Email us at care@somapeptides.com or schedule a telehealth appointment.